Sex- And Gender-based analysis of the Effectiveness of advanced therapies in Psoriatic Arthritis (SAGE-PsA Study)
Brief description of the SAGE Study:
The expansion of advanced targeted therapies has changed the way patients with PsA are treated. However, despite the effectiveness of these therapies, many patients do not achieve optimal response to these therapies. Sex and gender are important factors that influence treatment response in PsA. Women with PsA are less likely to achieve remission, are more likely to experience side effects and tend to stop treatments earlier than men.
SAGE-PsA is an international, multicenter study supported by GRAPPA through multi-pharma supported grants. The aim of the study is to understand how sex and gender influence response to advanced therapies in PsA. The investigators hope to discover biological and socio-cultural mechanisms that explain the differences in treatment response between men and women with PsA. The results of this study will improve our understanding of the impact of sex and gender on PsA which will contribute to more personalized approach to care of people living with PsA.
Study Principal Investigators

Study Steering Committee:
Primary Research Coordinator

FAHMEEN AFGANI, MBBS, CCRP
Women's College Hospital
Canada
Study Aims
The overarching aim of this SAGE-PsA is to understand how sex and gender, and their intersection with age and ethnicity, influence response to advanced therapies in PsA. Through sex & gender based analytic type framework, the investigators aim to uncover biological and socio-cultural mechanisms that underlie treatment disparities in PsA.
Specifically, the investigators aim to:
Study Population and procedures:
The study will prospectively enroll 540 men and women with active PsA from x countries across the world.
All participants will be starting treatment with 4 classes of targeted advanced therapies for peripheral musculoskeletal manifestations of PsA. Participants will be followed for a period of 1 year and will complete the following procedures:
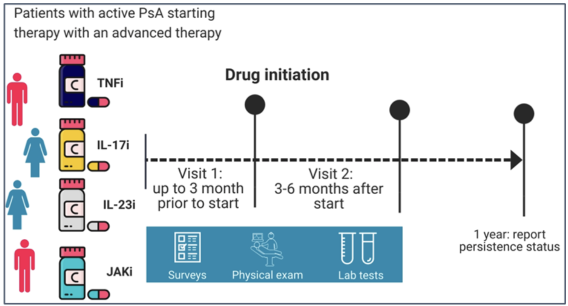
Study Sites & Co-Investigators:
The SAGE-PsA study will involve approximately 40 research sites worldwide. We are currently in the process of finalizing all participating sites for this study. The primary coordinating center is Women’s College Hospital, in Toronto, Canada.
Study outcomes:
SAGE-PsA will provide novel information about the role of sex and gender in PsA which should inform the development of more specific approaches to optimize the use of advanced therapies in people living with PsA.
Women's College Hospital
Canda
Dr. Lihi Eder
Swedish Medical Center
Canda
Philip Mease
Singapore General Hospital
Singapore
Katy Leung
Ottawa Hospital Research Institute
Canda
Sibel Aydin & Alexis Ogdie-Beatty
Toronto Western Hospital
Canda
Vinod Chandran
Hacettepe University, Ankara
Turkey
Umut Kalyoncu
Namik Kemal University
Turkey
Dilek Solmaz
University of São Paulo
Brazil
Claudia Schainberg
University of Molise
Italy
Ennio Lubrano
University of Oxford
UK
Laura Coates
Charité – Universitätsmedizin Berlin
Germany
Proft, Fabian Nikolai
Life Westville Hospital
South Africa
Ajesh Maharaj
Carmel Medical Center
Israel
Devy Zisman
Sengkang General Hospital
Singapore
Stanley Angkodjojo
The Chinese University of Hong Kong
China
Lai Shan Tam & Isaac Cheng
Christian Medical College, Vellore
India
Ashish Mathew
Kyorin University School of medicine
Japan
Mitsumasa Kishimoto
Fatima Memorial Hospital & FMH College of Medicine and Dentistry
Pakistan
Muhammad Haroon
Tel Aviv Medical Center
Israel
Ori Elkayam, Arik Polachek
Dr. Suliman Alhabib Hospital
United Arab Emirates
Ahmed Abogamal
Parker Institute
Denmark
Lars Erik
Pitie Salpétriere hospital and Sorbonne Université
France
Laure Gossec
IRCCS Humanitas Research Hospital
Italy
Carlo Selmi
Università Cattolica del Sacro Cuore, Fondazione Policlinico Gemelli IRCSS
Italy
Maria Antonietta D'Agostino & Dr. Ortolan
University of Cagliari
Italy
Alberto Cauli
Uppsala University Hospital
Sweden
Agnes Szentpetery
Cambridge University Hospitals
UK
Deepak Jadon
Hospital Italiano de Buenos Aires
Argentina
Maria Laura Acosta Felquer
Hospital de Clinicas Universidade Federal de Uberlandia
Brazil
Roberto Ranza
University Hospital Fundación Santa Fe de Bogotá
Colombia
Wilson Bautista-Molano
St. Paul Rheumatology
USA
David Ridley
Salt Lake City VA and University of Utah
USA
Jessie Walsh
UCSF
USA
Lianne Gensler
University of Florida Division of Rheumatology
USA
Gurjit Kaeley
UNIVERSITY OF CALIFORNIA DAVIS
USA
SIBA Raychaudhuri
University of Oregon
USA
Atul Deodhar
Rheumatology/Fraunhofer ITMP, University Hospital Frankfurt
Germany
Michaela Koehm
Study Timeline
FALL 2022
Secured funding
NOVEMBER 2022
REB approval at primary coordinating site (WCH, Toronto)
NOVEMBER 2022
Finalized 38 recruitment sites
CURRENT STAGE
Building REDCap database
CURRENT STAGE
Finalizing research contracts with sites
EXPECTED JANUARY 2023
Commencement of study recruitment
2023-2026
Data collection
EXPECTED 2026
Analysis and publication of results: anticipated 2026