The Diagnostic Ultrasound Enthesitis Tool (DUET) Study: Development of a Sonographic Enthesitis Score for Early Diagnosis of Psoriatic Arthritis
Brief description of the DUET Study:
Psoriatic arthritis (PsA) is a type of joint disease that can lead to severe joint damage and disability within the first few years of the disease. This is why early detection and treatment of the disease is essential to prevent serious joint damage and improve long-term outcomes in these patients. However, there is currently no reliable biomarker for PsA, which makes it difficult to detect PsA early. Enthesitis is an inflammation of the area where muscle tendons and ligaments attach to bones. Enthesitis is a key feature in PsA and can be easily detected using ultrasonography. The aim of this research study is to develop a system to evaluate enthesitis using ultrasonography, which can be used as an effective tool in the early detection of PsA. This will help in providing patients with early treatment to prevent further joint damage.
Study Principal Investigators

Primary Research Coordinator

Figure 1
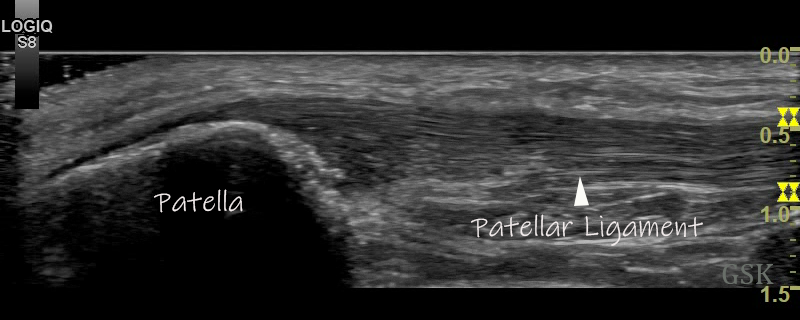
Figure 2
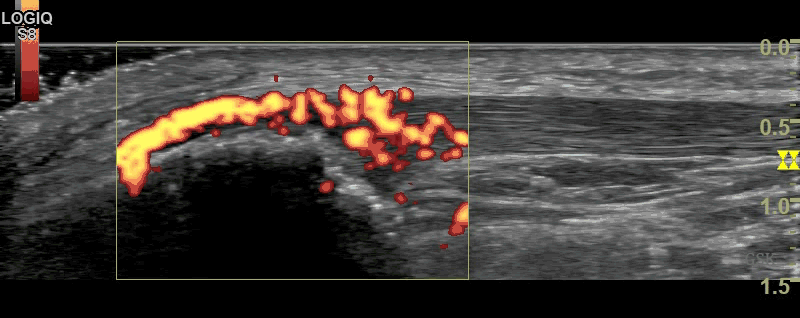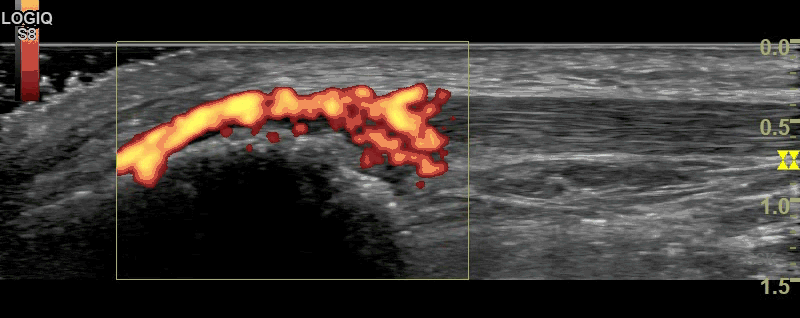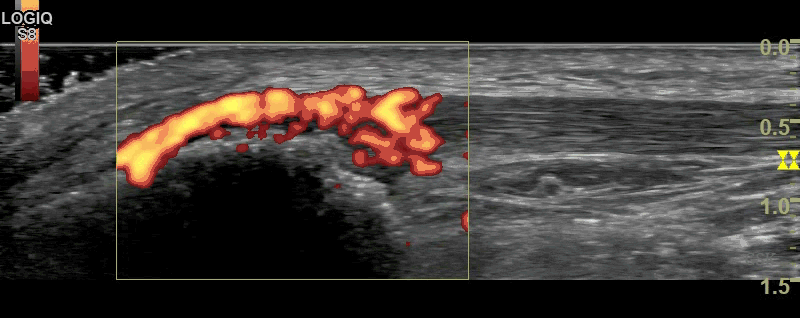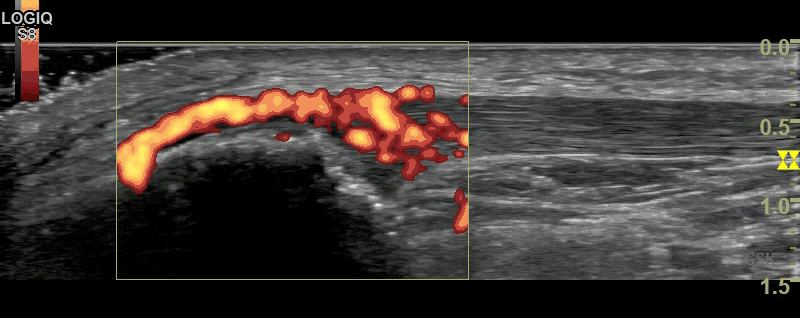
B-Mode (figure 1) and Power Doppler Scan (figure 2) of the proximal patellar ligament in a subject with psoriatic enthesopathy. Loss of Fibrillar echotexture is seen, which correlates to the maximal area of Doppler signal. Note fluffy bone formation at the bony margin.
Study Population:
A total of 400 participants will be recruited for this study, from the 3 following groups:
Study Procedures
All study participants will complete the following procedures:
Anticipated study outcome:
The study will provide a reliable and valid instrument for the identification of patients with PsA in rheumatology and dermatology settings. Our newly developed tool is expected to improve early detection of PsA by assisting the clinician to distinguish between PsA and other similar conditions. Early diagnosis should facilitate early interventions which will ultimately result in better control of disease activity and improve long term disease outcomes. Furthermore, we anticipate that the new standardized tool will also pave the way to more robust research in the PsA field, by allowing the conduction of ultrasound-based studies exploring approaches to improve the diagnosis and management of PsA.
Study Sites & Co-Investigators:
The DUET study has 21 research sites worldwide. The primary coordinating center is Women’s College Hospital, in Toronto, Canada.
Catharine Bakewell
Abha Singh
Sibel Aydin
Lihi Eder
Karen Salomon-Escoto
Minna Kohler Janeth Yinh
Gurjit Kaeley
Ernesto Rodriguez
Ricardo Acayaba
Mariana Alves Ferreira
Josefina Marin
Marcos Rosemffet
Agnes Szentpetery
Philippe Carron
Maria Stoenoiu
Ilaria Tinazzi
Amir Haddad
Ari Polachek
Basant Elnady
Study Timeline
MARCH 2020
Ethics Approval at Primary Coordinating Center
MARCH 2020
Finalized Study Sites Worldwide
SEPTEMBER 2020
Study Training Workshop
2021
Mutual Non-Disclosure Agreements with all sites
Local Ethics Submission & Approval at all study sites
Execution of Site Research Agreements
2021-2023
Study Recruitment and Data Collection
FEBRUARY 2023
Study Recruitment Closed
JUNE 2023
Study Completion
EXPECTED FALL 2023
Analysis & Publication of Results
Additional Resources
- Instructional videos demonstrating the recommended scanning techniques for the DUET Study.
- More information about this study can be found on the Clinicaltrials.gov website, registered under NCT04587362
*This study is supported by unrestricted research grants from Abbvie, Novartis, Janssen, Pfizer & Eli Lilly