Synovial tissue homing properties of novel monocyte subpopulations in active treatment naïve psoriatic arthritis.
Background:
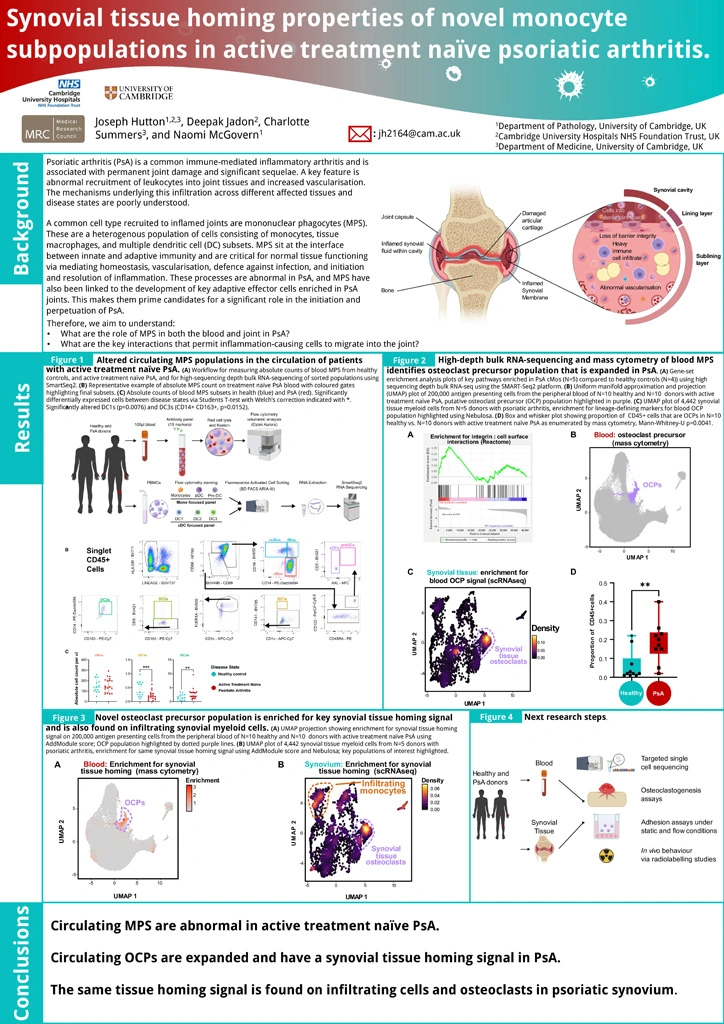
The recruitment of leukocytes into joint tissues is a key feature of psoriatic arthritis (PsA) yet the mechanisms underlying this infiltration across different tissues and disease states are poorly understood. Mononuclear phagocytes (MPS) are critical for vascularisation, and initiation/resolution of inflammation. These processes are abnormal in PsA, and MPS have also been linked to the production of key PsA cytokines and the development of key adaptive effector cells enriched in PsA.
Objective:
To identify the key interactions that drive MPS migration to the joint in active treatment-naïve PsA.
Methods:
Blood peripheral blood mononuclear cells were obtained from patients with active treatment-naïve PsA and healthy participants. High-sequencing depth bulk-sequencing using SmartSeq2 was performed on sort-purified monocyte and dendritic cell subsets from N=4 healthy and N=5 PsA donors. Gene set enrichment analysis was performed alongside expression of curated markers, comparing healthy and PsA subsets to identify key transcripts of interest. Gene expression changes were validated using multiparameter flow and mass cytometry. Observed changes in blood MPS populations where validated in PsA synovial tissue using publicly available single-cell RNA sequencing (scRNAseq) data.
Results:
Circulating MPS in PsA are enriched for transcripts that favour their adhesion, migration, and production of PsA-related cytokines. Mass cytometry identified a subset of circulating classical monocytes that are enriched for a osteoclast precursor (OCP) phenotype and are heavily enriched for markers of synovial tissue-homing. These tissue homing OCPs are significantly expanded in PsA. Infiltrating monocyte-derived synovial tissue macrophages (STMs) and osteoclasts (OC) in PsA express the same synovial tissue-homing markers.
Conclusions:
Our data suggests that circulating MPS are primed for synovial tissue homing. We identify a subset of circulating monocytes primed for tissue-homing, that is expanded in PsA and corresponds with joint-resident cells. Ongoing work will explore the functional outcome of these differences and trace their migration in vivo.