Patients developing inflammatory bowel disease before spondyloarthritis manifest significantly higher rates of enthesitis: findings from a single-center real-life cohort.
Authors
Keywords
entheropatic arthritis, enthesitis
Background.
Inflammatory bowel diseases (IBD) and spondyloarthritis (SpA) share numerous pathogenetic mechanisms and therapeutic options (i.e. biologics targeting TNFα and IL-23 inhibitors), especially in the case of psoriatic arthritis (PsA). Patients may present first with musculoskeletal or digestive manifestations and differences between these two phenotypes are unclear, particularly in terms of early presentations. From a clinical standpoint, subclinical enthesitis has been observed by ultrasonography in patients with IBD.
Objectives.
To analyze a cohort of patients diagnosed with both SpA/PsA and IBD and to compare the clinical features of patients presenting first with rheumatological manifestations (SpA>IBD) versus those developing IBD first (IBD>SpA).
Methods.
This is a retrospective study of patients with IBD and SpA/PsA being followed by a multidisciplinary team of gastroenterologists and rheumatologists at the Humanitas ImmunoCenter between March 2022 and March 2023. From all patients, demographic data and clinical characteristics including comorbidities, complications and therapeutic history were compared between SpA>IBD and IBD>SpA.
Results.
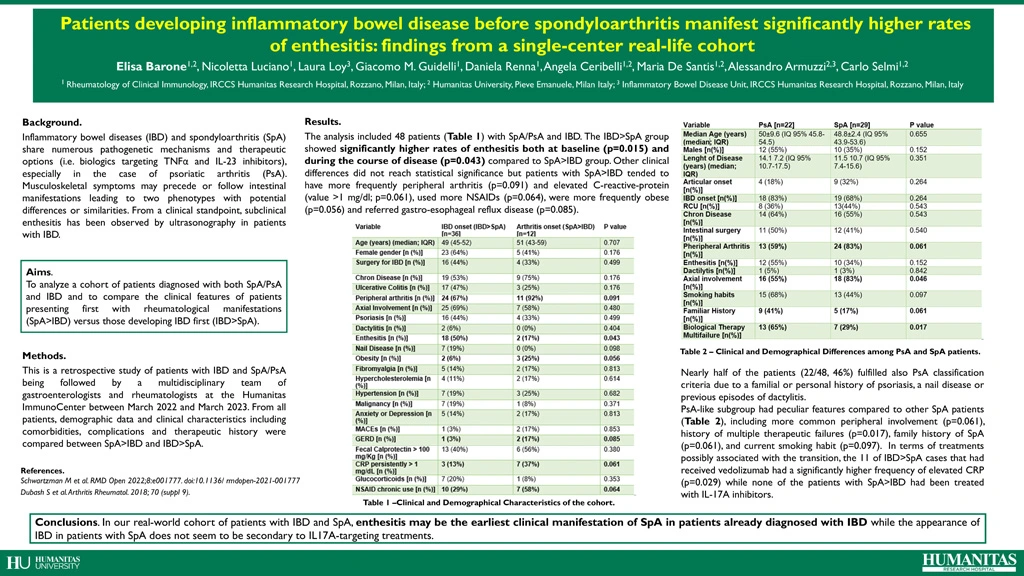
The analysis included 48 patients (Table 1) with SpA/PsA and IBD. Patients with IBD>SpA had significantly higher rates of enthesitis both at baseline (p=0.015) and during the course of disease (p=0.043) compared to SpA>IBD. Other clinical differences did not reach statistical significance but patients with SpA>IBD tended to have more frequently peripheral arthritis (p=0.091) and persistently elevated C-reactive-protein (CRP) >1 mg/dl (p=0.061), used more NSAIDs (p=0.064), were more frequently obese (p=0.056) and had more gastro-esophageal reflux disease (p=0.085). Nearly half of the patients (22/48, 46%) fulfilled also PsA classification criteria due to a familial or personal history of psoriasis, a nail disease or previous episodes of dactylitis. This PsA-like subgroup had peculiar features compared to other SpA patients, including more common peripheral involvement (p=0.061), history of multiple therapeutic failures (p=0.017), family history of SpA (p=0.061), and current smoking habit (p=0.097). In terms of treatments possibly associated with the transition, the 11 of IBD>SpA cases that had received vedolizumab had a significantly higher frequency of elevated CRP (p=0.029) while none of the patients with SpA>IBD had been treated with IL-17A inhibitors.
Conclusion.
In our real-world cohort of patients with IBD and SpA, enthesitis may be the earliest clinical manifestation of SpA in patients already diagnosed with IBD. The appearance of IBD in patients with SpA does not seem to be secondary to IL17A-targeting treatments.
| Variable | IBD onset (IBD>SpA) [n=36] | Arthritis onset (SpA>IBD) [n=12] | P value |
| Age (years) (median; IQR) | 49 (45-52) | 51 (43-59) | 0.707 |
| Female gender [n (%)] | 23 (64%) | 5 (41%) | 0.176 |
| Surgery for IBD [n (%)] | 16 (44%) | 4 (33%) | 0.499
|
| Chron Disease [n (%)] | 19 (53%) | 9 (75%) | 0.176 |
| Ulcerative Colitis [n (%)] | 17 (47%) | 3 (25%) | 0.176 |
| Peripheral arthritis [n (%)] | 24 (67%) | 11 (92%) | 0.091 |
| Axial Involvement [n (%)] | 25 (69%) | 7 (58%) | 0.480 |
| Psoriasis [n (%)] | 16 (44%) | 4 (33%) | 0.499 |
| Dactylitis [n (%)] | 2 (6%) | 0 (0%) | 0.404 |
| Enthesitis [n (%)] | 18 (50%) | 2 (17%) | 0.043 |
| Nail Disease [n (%)] | 7 (19%) | 0 (0%) | 0.098 |
| Obesity [n (%)] | 2 (6%) | 3 (25%) | 0.056 |
| Fibromyalgia [n (%)] | 5 (14%) | 2 (17%) | 0.813 |
| Hypercholesterolemia [n (%)] | 4 (11%) | 2 (17%) | 0.614 |
| Hypertension [n (%)] | 7 (19%) | 3 (25%) | 0.682 |
| Malignancy [n (%)] | 7 (19%) | 1 (8%) | 0.371 |
| Anxiety or Depression [n (%)] | 5 (14%) | 2 (17%) | 0.813 |
| MACEs [n (%)] | 1 (3%) | 2 (17%) | 0.853 |
| GERD [n (%)] | 1 (3%) | 2 (17%) | 0.085 |
| Fecal Calprotectin > 100 mg/Kg [n (%)] | 13 (40%) | 6 (56%) | 0.380 |
| CRP persistently > 1 mg/dL [n (%)] | 3 (13%) | 7 (37%) | 0.061 |
| Glucocorticoids [n (%)] | 7 (20%) | 1 (8%) | 0.353 |
| NSAID chronic use [n (%)] | 10 (29%) | 7 (58%) | 0.064 |
Table 1. Clinical and Demographic Characteristics of the cohort.
| Variable | PsA [n=22] | SpA [n=29] | P value |
| Median Age (years) (median; IQR) | 50±9.6 (IQ 95% 45.8-54.5) | 48.8±2.4 (IQ 95% 43.9-53.6) | 0.655 |
| Males [n(%)] | 12 (55%) | 10 (35%) | 0.152 |
| Lenght of Disease (years) (median; IQR) | 14.1 7.2 (IQ 95% 10.7-17.5) | 11.5 10.7 (IQ 95% 7.4-15.6) | 0.351 |
| Articular onset [n(%)] | 4 (18%) | 9 (32%) | 0.264 |
| IBD onset [n(%)] | 18 (83%) | 19 (68%) | 0.264 |
| RCU [n(%)] | 8 (36%) | 13(44%) | 0.543 |
| Chron Disease [n(%)] | 14 (64%) | 16 (55%) | 0.543 |
| Intestinal surgery [n(%)] | 11 (50%) | 12 (41%) | 0.540 |
| Pheripheral Arthritis [n(%)] | 13 (59%) | 24 (83%) | 0.061 |
| Enthesitis [n(%)] | 12 (55%) | 10 (34%) | 0.152 |
| Dactilytis [n(%)] | 1 (5%) | 1 (3%) | 0.842 |
| Axial involvement [n(%)] | 16 (55%) | 18 (83%) | 0.046 |
| Smoking habits [n(%)] | 15 (68%) | 13 (44%) | 0.097 |
| Familiar History [n(%)] | 9 (41%) | 5 (17%) | 0.061 |
| Biological Therapy Multifailure [n(%)] | 13 (65%) | 7 (29%) | 0.017 |
Table 2. Clinical and Demographical Differences among PsA and SpA patients.