A cytokine signature from monocyte-derived macrophages predicts response to apremilast in patients with psoriatic arthritis
Authors
Keywords
apremilast; IL-23 signature; macrophages; myeloid
Background. The immune mechanisms of action remain elusive for apremilast, a PDE4-inhibitor approved for the treatment of psoriatic arthritis (PsA), although data support the regulation of innate immune cells, particularly macrophages. The DAPSA score is accepted for the evaluation of treatment response and subdivides patients into low, moderate, and high disease activity.
Objective. To investigate the ex vivo effects of apremilast on monocyte-derived macrophages in PsA patients.
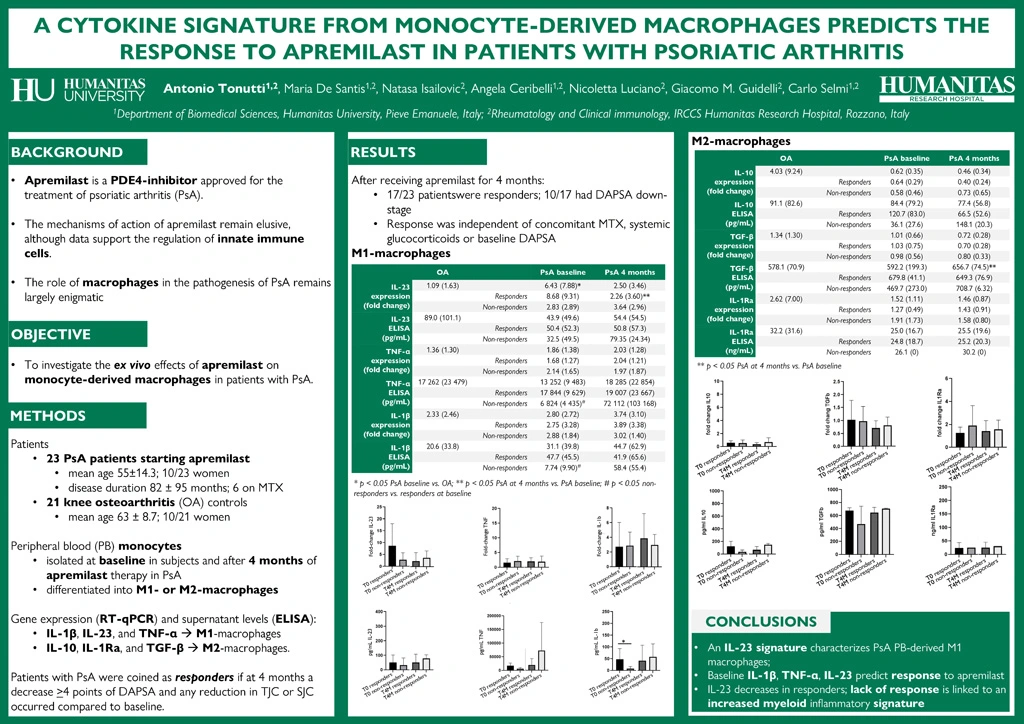
Methods. We enrolled twenty-three PsA patients starting apremilast (mean age 55±14.3; 10/23 women; disease duration 82±95 months, 6 on methotrexate) while 21 patients with knee osteoarthritis (OA) served as controls (age 63±8.7, p=0.05; 10/21 women). Peripheral blood monocytes were isolated at baseline in all patients and after 4 months of therapy in PsA. After differentiation into M1- or M2-macrophages, gene expression (RT-qPCR) and supernatant levels (ELISA) of IL-1β, IL-23, and TNF-α were analyzed in M1-macrophages, while IL-10, IL-1Ra, and TGF-β were arrayed in M2-macrophages. Patients with PsA were considered as responders if at 4 months a decrease ³4 points of DAPSA and any reduction in tender and swollen joint counts occurred.
Results. At 4 months, 17/23 (74%) patients were responders (10/23 had a DAPSA reduction from high to moderate or from moderate to low disease activity). Response to apremilast was not influenced by concomitant methotrexate (3/17 responders vs. 3/6 non-responders), systemic glucocorticoids (used by 3 patients, 1 responder), or baseline DAPSA (16.8±12.5 vs. 15.0±2.8).
In the analysis of M1-macrophages, baseline IL-23 expression was higher in PsA than OA (p=0.003), while the expression and surnatant levels of TNF-α and IL-1β did not differ. Responder PsA cases had non-significantly higher baseline IL-23 and significantly higher TNF-α and IL-1β (p=0.03) compared to non-responders. At 4 months, IL-23 expression decreased in responders (p=0.031), while inflammatory cytokines increased in non-responders (p=ns).
When M2-macrophages were analyzed, baseline IL-10 and IL-1Ra levels were higher in OA while in PsA there was a significant increase in TGF-β supernatant levels at 4 months (p=0.023), possibly related to enhanced cleavage by macrophage stimulation as no expression increase was observed. Response to apremilast was not associated to changes in regulatory cytokines.
Conclusions. An IL-23 signature characterizes monocyte-derived M1-macrophages in PsA, and high baseline levels of IL-1β, TNF-α, and IL-23 are associated with the response to apremilast. There is a decrease in IL-23 expression in responders to apremilast, while the lack of response is linked to an increased myeloid inflammatory signature.